Isotonic Solution Definition
Isotonic solutions are those with the same solute concentration, or osmolarity, as another solution. The water will flow in equal amounts out of each solution and into the other if the two solutions are separated by a semipermeable membrane. As a result, there is no water flow between the two solutions, even though water is moving both ways.
For cellular functions to be sustained, some cells need to be maintained in an isotonic solution. Animal cells, which lack a cell wall to protect them from water pressure, rely on the stability of the external environment to maintain their shape.
To bathe their cells in isotonic solutions, animals maintain the pH and osmolarity of their body fluids. Nutrients and water can be carried by this solution, but only in proportion to the amount inside the cell.
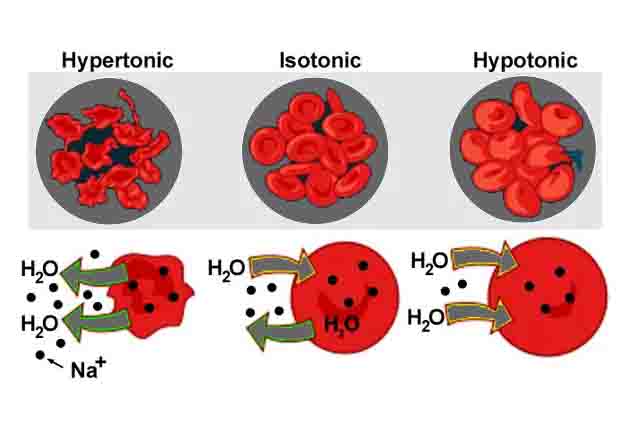
In the image above, you can see an isotonic solution depicting a cell. Due to the same concentration of solute molecules inside and outside of the cell, water molecules are simply exchanged through the cell membrane. A hypertonic solution, in which water molecules leave the cell, or a hypotonic solution, in which water enters, can be contrasted with this effect.
Examples of Isotonic Solution
Blood Cells
Blood cells function normally when the plasma surrounding them is isotonic compared to the solution inside them. As a result of the isotonic solution, water and nutrients can move in and out of the cells. For blood cells to deliver oxygen and other nutrients to other parts of the body, this is necessary.
In hypertonic environments, the cells become plasmolyzed and do not contain enough water to perform cellular functions. A hypotonic environment will cause the cells to lyse, spilling their contents into the bloodstream. In addition to causing dangerous side effects, this can lead to the loss of many blood cells.
During the transfusion of nutrients and medicine, the solution that carries the medicine should be an isotonic solution, compared to the patient’s blood. Salts and sugars that act as solutes to dilute or strengthen a substance can be used to adjust the osmolarity of IV fluid. Medicine added through an IV will not damage blood cells once it is an isotonic solution compared to the blood.
Osmoconformers and Osmoregulators
Two types of organisms exist in nature: those that conform to the osmolarity of their environment, and those that regulate their osmolarity to be different. First, there are osmoconformers, which have evolved to have cells that match the osmolarity of the environment.
Since these animals have evolved to be the same concentration as their environment, they always exist in an isotonic solution. There are many “lower” forms of life that exhibit this condition, such as sea slugs, corals, and jellyfish. As for the osmoregulators, they do not exist in an isotonic environment.
As a result, water tends to enter or leave their bodies in a variety of ways. The cells inside an osmoregulator will, however, still exist in an isotonic solution, since the organism needs its cells to function. As osmoregulators and osmoconformers conduct life differently, an isotonic solution is usually created around cells.
Related Biology Terms
- Hypotonic – When a solution has comparatively more water and less solute.
- Hypertonic – A solution with less water and more solute than another solution.
- Osmolarity – The overall solute concentration of a solution.